NMR structure of growth factor receptor binding protein SH2 domain complexed with the inhibitor
Ogura, K., Shiga, T., Yuzawa, S., Yokochi, M., Burke, T.R., Inagaki, F.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Growth factor receptor-bound protein 2 | 104 | Homo sapiens | Mutation(s): 0 | 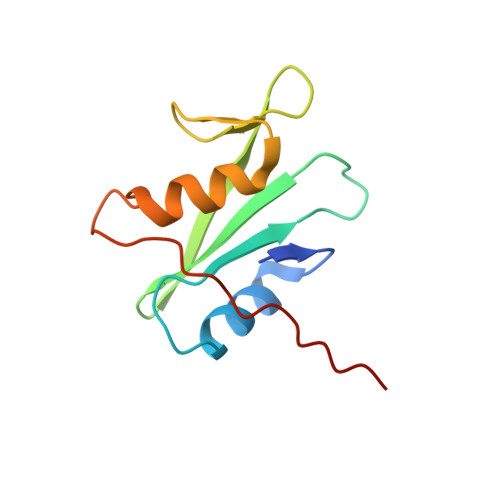 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P62993 (Homo sapiens) Explore P62993 Go to UniProtKB: P62993 | |||||
PHAROS: P62993 GTEx: ENSG00000177885 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P62993 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| DTF Query on DTF | B [auth A] | 4-[(10S,14S,18S)-18-(2-AMINO-2-OXOETHYL)-14-(1-NAPHTHYLMETHYL)-8,17,20-TRIOXO-7,16,19-TRIAZASPIRO[5.14]ICOS-11-EN-10-YL]BENZYLPHOSPHONIC ACID C37 H45 N4 O7 P RHYFMOCFCFUTNH-GZNVFMSSSA-N |  | ||