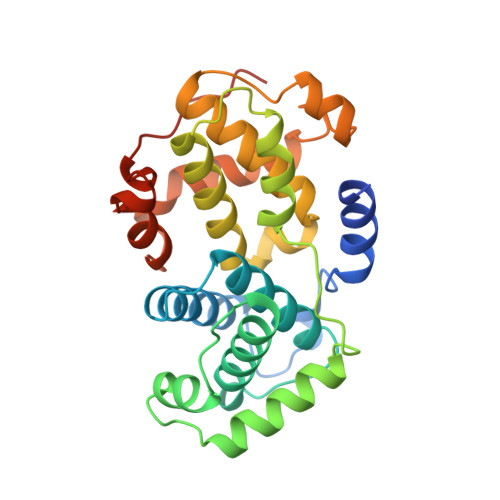
The crystal structure of cyclin A.
Brown, N.R., Noble, M.E., Endicott, J.A., Garman, E.F., Wakatsuki, S., Mitchell, E., Rasmussen, B., Hunt, T., Johnson, L.N.(1995) Structure 3: 1235-1247
- PubMed: 8591034
- DOI: https://doi.org/10.1016/s0969-2126(01)00259-3
- Primary Citation of Related Structures:
1VIN - PubMed Abstract:
Eukaryotic cell cycle progression is regulated by cyclin dependent protein kinases (CDKs) whose activity is regulated by association with cyclins and by reversible phosphorylation. Cyclins also determine the subcellular location and substrate specificity of CDKs. Cyclins exhibit diverse sequences but all share homology over a region of approximately 100 amino acids, termed the cyclin box. From the determination of the structure of cyclin A, together with results from biochemical and genetic analyses, we can identify which parts of the cyclin molecular may contribute to cyclin A structure and function.
Organizational Affiliation:
Laboratory of Molecular Biophysics, Oxford, UK.