Novel purine nitrile derived inhibitors of the cysteine protease cathepsin K
Altmann, E., Cowan-Jacob, S.W., Missbach, M.(2004) J Med Chem 47: 5833-5836
- PubMed: 15537340
- DOI: https://doi.org/10.1021/jm0493111
- Primary Citation of Related Structures:
1U9V, 1U9W, 1U9X - PubMed Abstract:
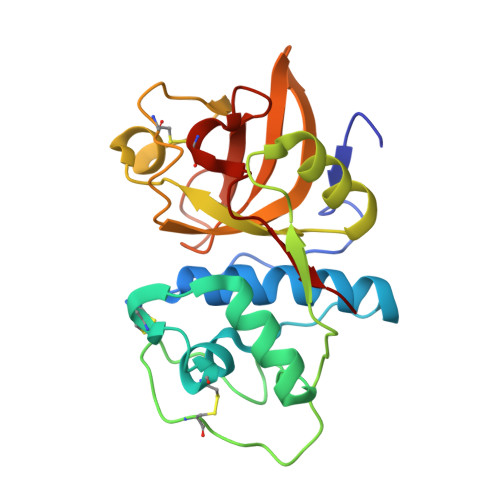
Starting from the high-throughput screening hit 1a, novel cathepsin K inhibitors have been developed based on a purine scaffold. High-resolution X-ray structures of several derivatives have revealed the binding mode of these unique cysteine protease inhibitors.
Organizational Affiliation:
Novartis Institutes for BioMedical Research, CH-4002 Basel, Switzerland. eva.altmann@pharma.novartis.com