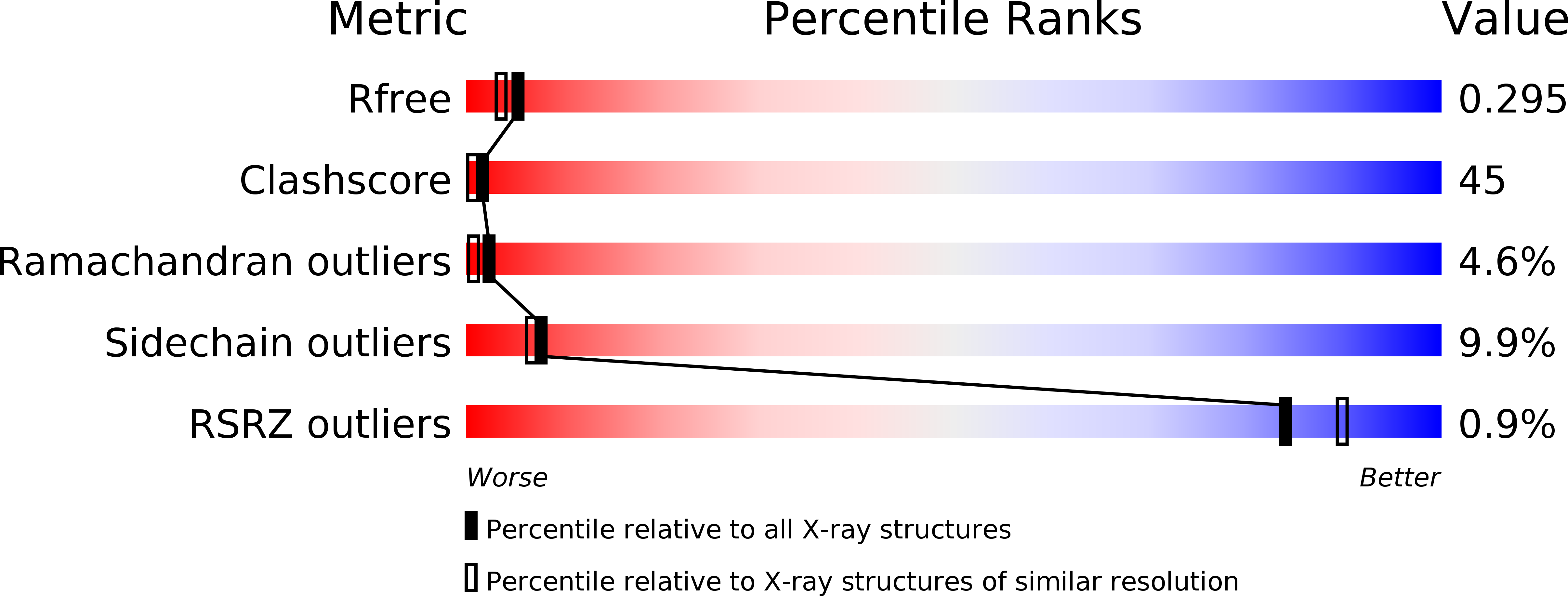
Crystal structure of the spindle assembly checkpoint protein Bub3
Larsen, N.A., Harrison, S.C.(2004) J Mol Biol 344: 885-892
- PubMed: 15544799
- DOI: https://doi.org/10.1016/j.jmb.2004.09.094
- Primary Citation of Related Structures:
1U4C - PubMed Abstract:
Bub3 is one of at least six proteins that transmit the spindle assembly checkpoint signal. These proteins delay cell cycle progression from metaphase to anaphase in response to attachment defects between kinetochores and spindle microtubules and to tension defects between sister chromatids. To explore the molecular interactions mediated by Bub3, we have determined the crystal structure of the Saccharomyces cerevisiae protein Bub3p at 2.35 A resolution. Bub3p is a seven-blade beta-propeller, although its sequence diverges from that of other WD40 family members. Several loops are substantially elongated, but extra domains or insertions are not present at the termini. In particular, two extended loops project from the top face of the propeller, forming a cleft. Amino acid residues across the top face and one aspect of the lateral surface (spanning blades 5-6) are highly conserved among Bub3 proteins. We propose that these conserved surfaces are the loci for key interactions with conserved motifs in spindle checkpoint proteins Bub1 and Mad3/BubR1. Comparison of the Bub3 sequence to the WD40 protein, Rae1, shows high sequence conservation along the same surfaces. Rae1 interaction with Bub1 is, therefore, likely to involve a similar mode of binding.
Organizational Affiliation:
Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA, USA.