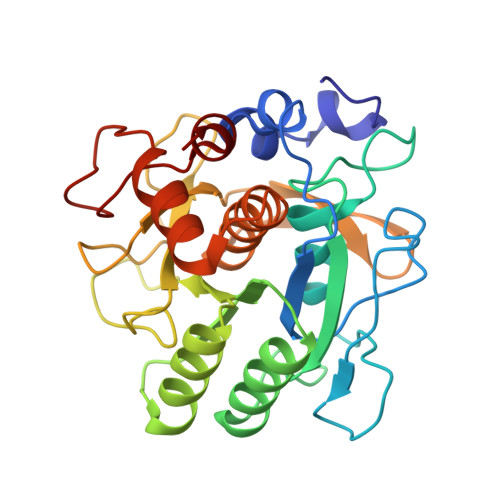
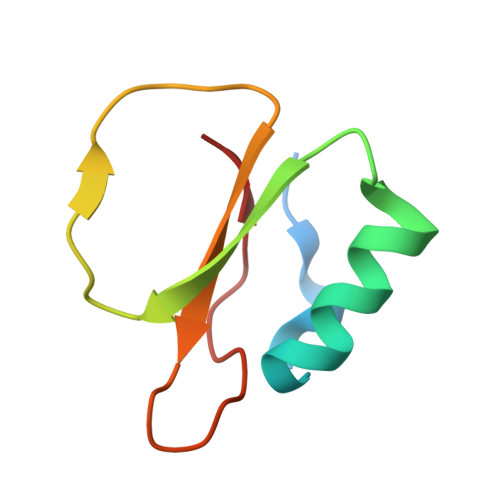
Crystallographic refinement by incorporation of molecular dynamics: thermostable serine protease thermitase complexed with eglin c.
Gros, P., Fujinaga, M., Dijkstra, B.W., Kalk, K.H., Hol, W.G.(1989) Acta Crystallogr B 45: 488-499
- PubMed: 2688688
- DOI: https://doi.org/10.1107/s0108768189006038
- Primary Citation of Related Structures:
1TEC - PubMed Abstract:
In order to investigate the principles of protein thermostability, the crystal structure of thermitase from Thermoactinomyces vulgaris, a thermostable member of the subtilisin family of serine proteases, has been determined in a complex with eglin c. Eglin c is a serine protease inhibitor from the leech Hirudo medicinalis. After data collection with a television area-detector diffractometer and initial structure solution by molecular-replacement methods, crystallographic refinement proceeded with incorporation of molecular-dynamics techniques. It appeared that this refinement procedure has a large convergence radius with movements of more than 5 A for many atoms. Two procedures for the crystallographic molecular-dynamics refinement have been tested. They differed mainly in time span and weight on the X-ray 'energy'. The best results were obtained with a procedure which allowed the molecular-dynamics technique to search a large area in conformational space by having less weight on the X-ray restraints and allowing more time. The use of molecular-dynamics refinement considerably simplified the laborious and difficult task of fitting the model in its electron density during the refinement process. The final crystallographic R factor is 17.9% at 2.2 A resolution.
Organizational Affiliation:
Department of Chemistry, University of Groningen, The Netherlands.