Three-Dimensional Structure in Lipid Micelles of the Pediocin-Like Antimicrobial Peptide Sakacin P and a Sakacin P Variant that is Structurally Stabilized by an Inserted C-Terminal Disulfide Bridge
Uteng, M., Hauge, H.H., Markwick, P.R., Fimland, G., Mantzilas, D., Nissen-Meyer, J., Muhle-Goll, C.(2003) Biochemistry 42: 11417
- PubMed: 14516192
- DOI: https://doi.org/10.1021/bi034572i
- Primary Citation of Related Structures:
1OG7, 1OHM, 1OHN - PubMed Abstract:
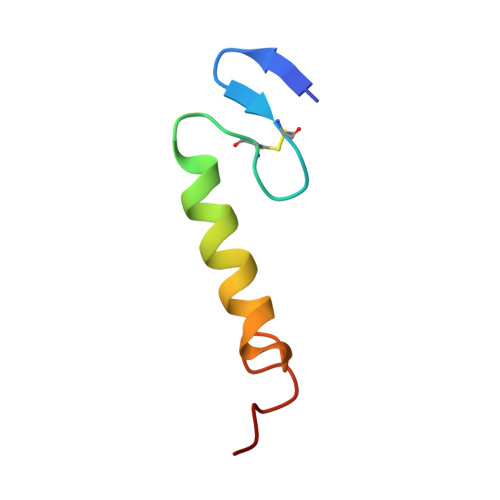
The three-dimensional structures in dodecylphosphocholine (DPC) micelles and in trifluoroethanol (TFE) of the pediocin-like antimicrobial peptide sakacin P and an engineered variant of sakacin P (termed sakP[N24C+44C]) have been determined by use of nuclear magnetic resonance spectroscopy. SakP[N24C+44C] has an inserted non-native activity- and structure-stabilizing C-terminal disulfide bridge that ties the C-terminus to the middle part of the peptide. In the presence of DPC, the cationic N-terminal region (residues 1-17) of both peptides has an S-shaped conformation that is reminiscent of a three-stranded antiparallel beta-sheet and that is more pronounced when the peptide was dissolved in TFE instead of DPC. The four positively charged residues located in the N-terminal part are found pointing to the same direction. For both peptides, the N-terminal region is followed by a well-defined central amphiphilic alpha-helix (residues 18-33), and this in turn is followed by the C-terminal tail (residues 34-43 for sakacin P and 34-44 for sakP[N24C+44C]) that lacks any apparent common secondary structural motif. In the presence of DPC, the C-terminal tails in both peptides fold back onto the central alpha-helix, thereby creating a hairpin-like structure in the C-terminal halves. The lack of long-range NOEs between the beta-sheet Nu-terminal region and the hairpin-like C-terminal half indicates that there is a flexible hinge between these regions. We discuss which implications such a structural arrangement has on the interaction with the target cell membrane.
Organizational Affiliation:
Department of Biochemistry, University of Oslo, Oslo, Norway.