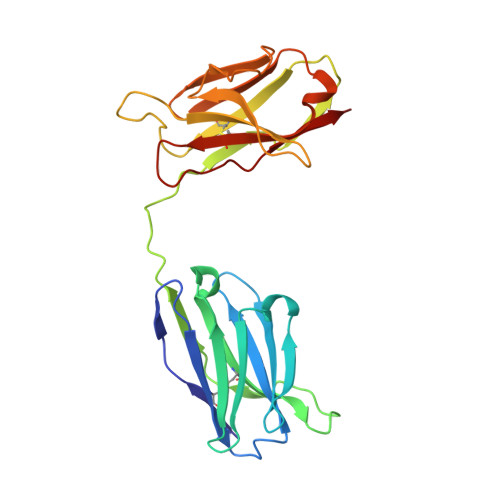
Crystal structure of a diabody, a bivalent antibody fragment.
Perisic, O., Webb, P.A., Holliger, P., Winter, G., Williams, R.L.(1994) Structure 2: 1217-1226
- PubMed: 7704531
- DOI: https://doi.org/10.1016/s0969-2126(94)00123-5
- Primary Citation of Related Structures:
1LMK - PubMed Abstract:
Diabodies are dimeric antibody fragments. In each polypeptide, a heavy-chain variable domain (VH) is linked to a light-chain variable domain (VL) but unlike single-chain Fv fragments, each antigen-binding site is formed by pairing of one VH and one VL domain from the two different polypeptides. Diabodies thus have two antigen-binding sites, and can be bispecific. Direct structural evidence is lacking for the connections and dimeric interactions between the two polypeptides of the diabody.
Organizational Affiliation:
Centre for Protein Engineering, MRC Centre, Cambridge, UK.