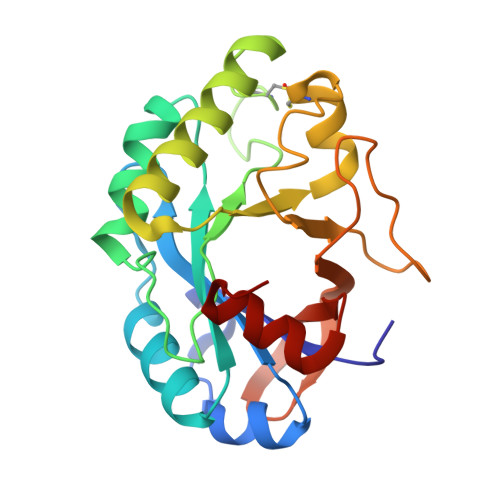
A new lysozyme fold. Crystal structure of the muramidase from Streptomyces coelicolor at 1.65 A resolution.
Rau, A., Hogg, T., Marquardt, R., Hilgenfeld, R.(2001) J Biol Chem 276: 31994-31999
- PubMed: 11427528
- DOI: https://doi.org/10.1074/jbc.M102591200
- Primary Citation of Related Structures:
1JFX - PubMed Abstract:
Cellosyl is a bacterial muramidase from Streptomyces coelicolor. Similar to other lysozymes, the enzyme cleaves the beta-1,4-glycosidic bond between N-acetylmuramic acid and N-acetylglucosamine units, but it also exhibits a beta-1,4-N,6-O-diacetylmuramidase activity. The latter enables Cellosyl to degrade the cell walls of Staphylococcus aureus, which are not hydrolyzed by chicken-, goose-, or bacteriophage T4-type lysozymes. The enzymatic activity and amino acid sequence of Cellosyl group it with lysozymes of the Chalaropsis type, for which no detailed structural information has been available so far. The crystal structure of Cellosyl from S. coelicolor has been determined to a resolution of 1.65 A and refined to an R-factor of 15.2%. The enzyme is comprised of a single domain and possesses an unusual beta/alpha-barrel fold. The last strand, beta 8, of the (beta/alpha)(5)beta(3)-barrel is found to be antiparallel to strands beta 7 and beta 1. Asp-9, Asp-98, and Glu-100 are located at the active site. The structure of Cellosyl exhibits a new lysozyme fold and represents a new class of polysaccharide-hydrolyzing beta/alpha-barrels.
Organizational Affiliation:
Department of Structural Biology & Crystallography, Institute of Molecular Biotechnology, Beutenbergstrasse 11, 07745 Jena, Germany.