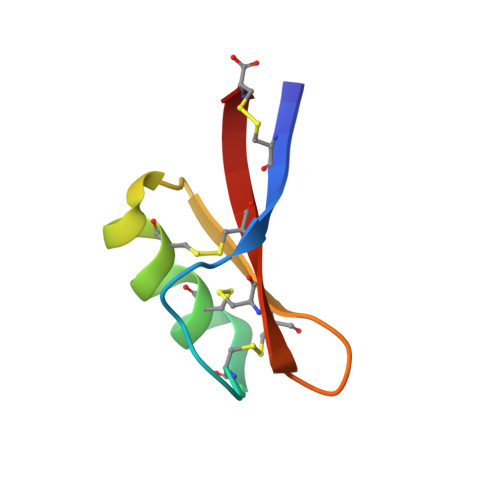
Solution structure of gamma 1-H and gamma 1-P thionins from barley and wheat endosperm determined by 1H-NMR: a structural motif common to toxic arthropod proteins.
Bruix, M., Jimenez, M.A., Santoro, J., Gonzalez, C., Colilla, F.J., Mendez, E., Rico, M.(1993) Biochemistry 32: 715-724
- PubMed: 8380707
- DOI: https://doi.org/10.1021/bi00053a041
- Primary Citation of Related Structures:
1GPS, 1GPT - PubMed Abstract:
The complete assignment of the proton NMR spectra of the homologous gamma 1-hordothionin and gamma 1-purothionin (47 amino acids, 4 disulfide bridges) from barley and wheat, respectively, has been performed by two-dimensional sequence-specific methods. A total of 299 proton-proton distance constraints for gamma 1-H and 285 for gamma 1-P derived from NOESY spectra have been used to calculate the three-dimensional solution structures. Initial structures have been generated by distance geometry methods and further refined by dynamical simulated annealing calculations. Both proteins show identical secondary and tertiary structure with a well-defined triple-stranded antiparallel beta-sheet (residues 1-6, 31-34, and 39-47), an alpha-helix (residues 16-28), and the corresponding connecting loops. Three disulfide bridges are located in the hydrophobic core holding together the alpha-helix and the beta-sheet and forming a cysteine-stabilized alpha-helical (CSH) motif. Moreover, a clustering of positive charges is observed on the face of the beta-sheet opposite to the helix. The three-dimensional structures of the gamma-thionins differ remarkably from plant alpha- and beta-thionins and crambin. However, they show a higher structural analogy with scorpion toxins and insect defensins which also present the CSH motif.
Organizational Affiliation:
Instituto de Estructura de la Materia, CSIC, Madrid, Spain.