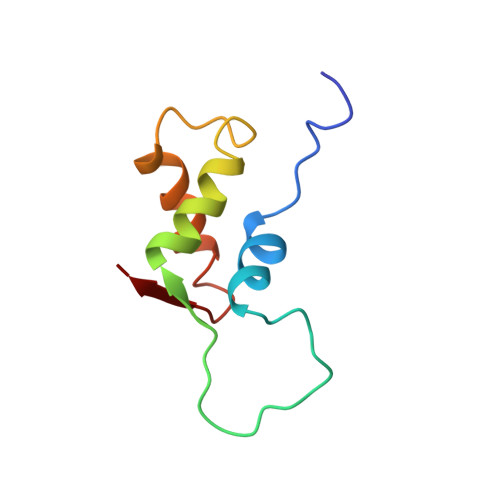
High resolution solution structure of ribosomal protein L11-C76, a helical protein with a flexible loop that becomes structured upon binding to RNA.
Markus, M.A., Hinck, A.P., Huang, S., Draper, D.E., Torchia, D.A.(1997) Nat Struct Biol 4: 70-77
- PubMed: 8989327
- DOI: https://doi.org/10.1038/nsb0197-70
- Primary Citation of Related Structures:
1FOW, 1FOX - PubMed Abstract:
The structure of the C-terminal RNA recognition domain of ribosomal protein L11 has been solved by heteronuclear three-dimensional nuclear magnetic resonance spectroscopy. Although the structure can be considered high resolution in the core, 15 residues between helix alpha 1 and strand beta 1 form an extended, unstructured loop. 15N transverse relaxation measurements suggest that the loop is moving on a picosecond-to-nanosecond time scale in the free protein but not in the protein bound to RNA. Chemical shifts differences between the free protein and the bound protein suggest that the loop as well as the C-terminal end of helix alpha 3 are involved in RNA binding.
Organizational Affiliation:
Molecular Structural Biology Unit, National Institute of Dental Research, Bethesda, Maryland 20892-4320, USA.