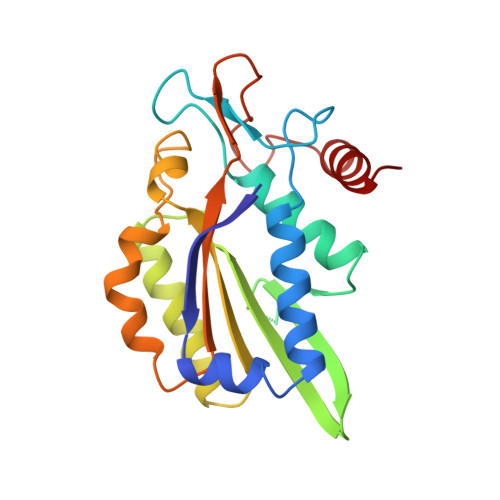
Structure of restriction endonuclease bamhi phased at 1.95 A resolution by MAD analysis.
Newman, M., Strzelecka, T., Dorner, L.F., Schildkraut, I., Aggarwal, A.K.(1994) Structure 2: 439-452
- PubMed: 8081758
- DOI: https://doi.org/10.1016/s0969-2126(00)00045-9
- Primary Citation of Related Structures:
1BAM - PubMed Abstract:
Type II restriction endonucleases recognize DNA sequences that vary between four to eight base pairs, and require only Mg2+ as a cofactor to catalyze the hydrolysis of DNA. Their protein sequences display a surprising lack of similarity, and no recurring structural motif analogous to the helix-turn-helix or the zinc finger of transcription factors, has yet been discovered.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, Columbia University, New York, NY 10032.