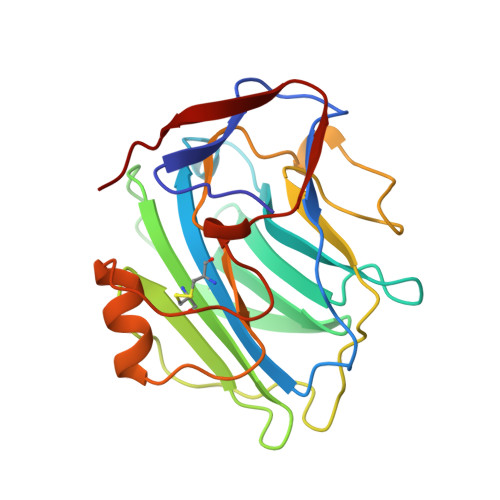
The physiological structure of human C-reactive protein and its complex with phosphocholine.
Thompson, D., Pepys, M.B., Wood, S.P.(1999) Structure 7: 169-177
- PubMed: 10368284
- DOI: https://doi.org/10.1016/S0969-2126(99)80023-9
- Primary Citation of Related Structures:
1B09 - PubMed Abstract:
Human C-reactive protein (CRP) is the classical acute phase reactant, the circulating concentration of which rises rapidly and extensively in a cytokine-mediated response to tissue injury, infection and inflammation. Serum CRP values are routinely measured, empirically, to detect and monitor many human diseases. However, CRP is likely to have important host defence, scavenging and metabolic functions through its capacity for calcium-dependent binding to exogenous and autologous molecules containing phosphocholine (PC) and then activating the classical complement pathway. CRP may also have pathogenic effects and the recent discovery of a prognostic association between increased CRP production and coronary atherothrombotic events is of particular interest.
Organizational Affiliation:
School of Biological Sciences, University of Southampton, Southampton SO16 7PX UK.