Structural basis for bivalent smac-mimetics recognition in the IAP protein family
Cossu, F., Milani, M., Mastrangelo, E., Vachette, P., Servida, F., Lecis, D., Canevari, G., Delia, D., Drago, C., Rizzo, V., Manzoni, L., Seneci, P., Scolastico, C., Bolognesi, M.(2009) J Mol Biol 392: 630-644
- PubMed: 19393243
- DOI: https://doi.org/10.1016/j.jmb.2009.04.033
- Primary Citation of Related Structures:
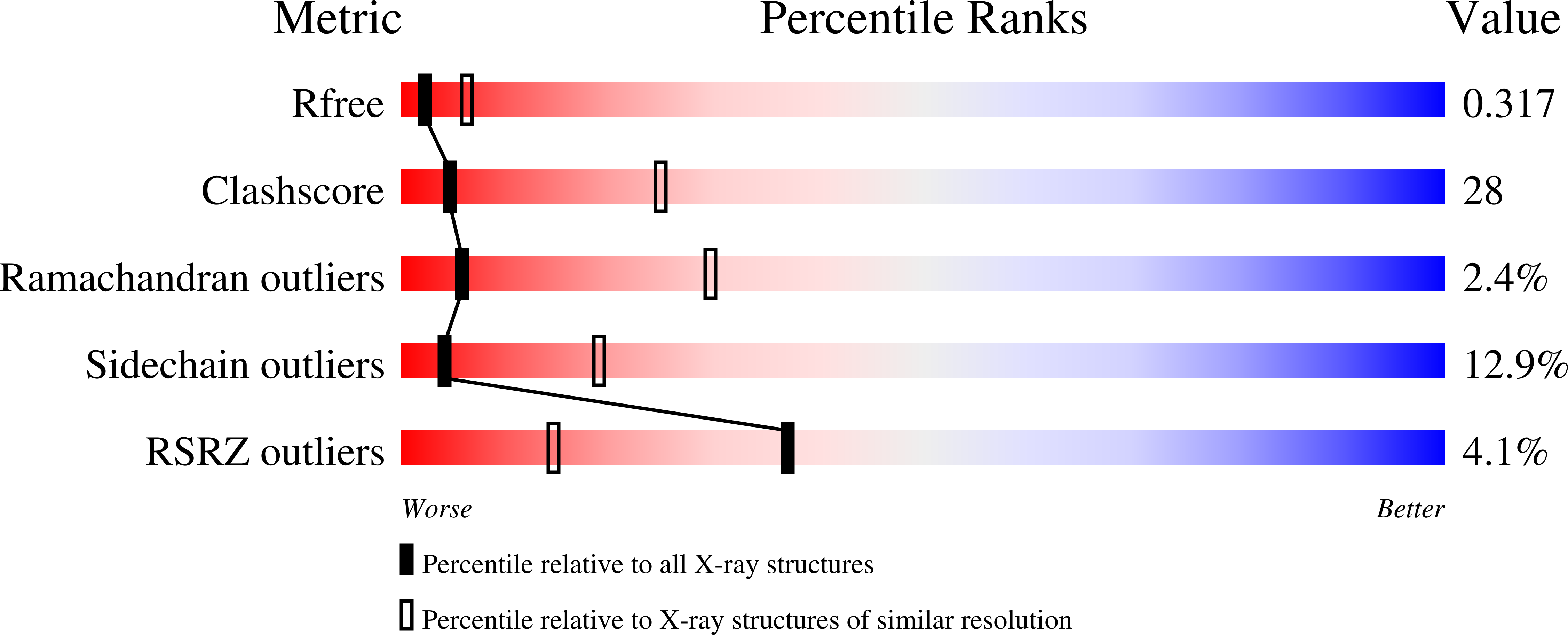
3G76 - PubMed Abstract:
XIAP is an apoptotic regulator protein that binds to the effector caspases -3 and -7 through its BIR2 domain, and to initiator caspase-9 through its BIR3 domain. Molecular docking studies suggested that Smac-DIABLO may antagonize XIAP by concurrently targeting both BIR2 and BIR3 domains; on this basis bivalent Smac-mimetic compounds have been proposed and characterized. Here, we report the X-ray crystal structure of XIAP-BIR3 domain in complex with a two-headed compound (compound 3) with improved efficacy relative to its monomeric form. A small-angle X-ray scattering study of XIAP-BIR2BIR3, together with fluorescence polarization binding assays and compound 3 cytotoxicity tests on HL60 leukemia cell line are also reported. The crystal structure analysis reveals a network of interactions supporting XIAP-BIR3/compound 3 recognition; moreover, analytical gel-filtration chromatography shows that compound 3 forms a 1:1 stoichiometric complex with a XIAP protein construct containing both BIR2 and BIR3 domains. On the basis of the crystal structure and small-angle X-ray scattering, a model of the same BIR2-BIR3 construct bound to compound 3 is proposed, shedding light on the ability of compound 3 to relieve XIAP inhibitory effects on caspase-9 as well as caspases -3 and -7. A molecular modeling/docking analysis of compound 3 bound to cIAP1-BIR3 domain is presented, considering that Smac-mimetics have been shown to kill tumor cells by inducing cIAP1 and cIAP2 ubiquitination and degradation. Taken together, the results reported here provide a rationale for further development of compound 3 as a lead in the design of dimeric Smac mimetics for cancer treatment.
Organizational Affiliation:
Department of Biomolecular Sciences and Biotechnology, University of Milano, Italy.