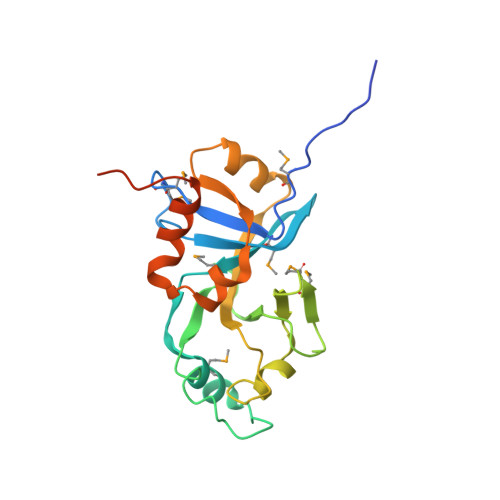
X-Ray structure of hypothetical protein VC0467 from Vibrio cholerae: new fold. Northeast Structural Genomics Consortium target VcR8.
Kuzin, A.P., Abashidze, M., Vorobiev, S.M., Acton, T., Xiao, R., Conover, K., Ma, L.-C., Kellie, R., Montelione, G.T., Hunt, J.F., Tong, L.To be published.