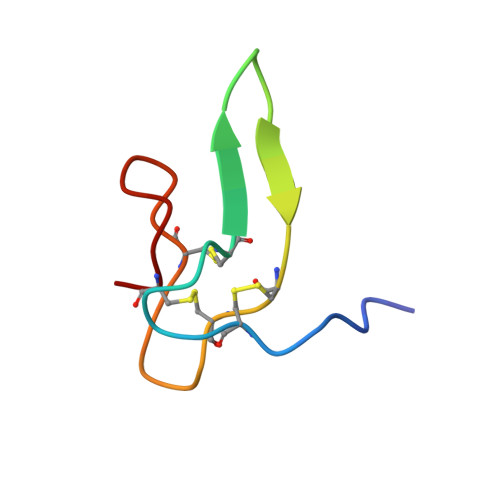
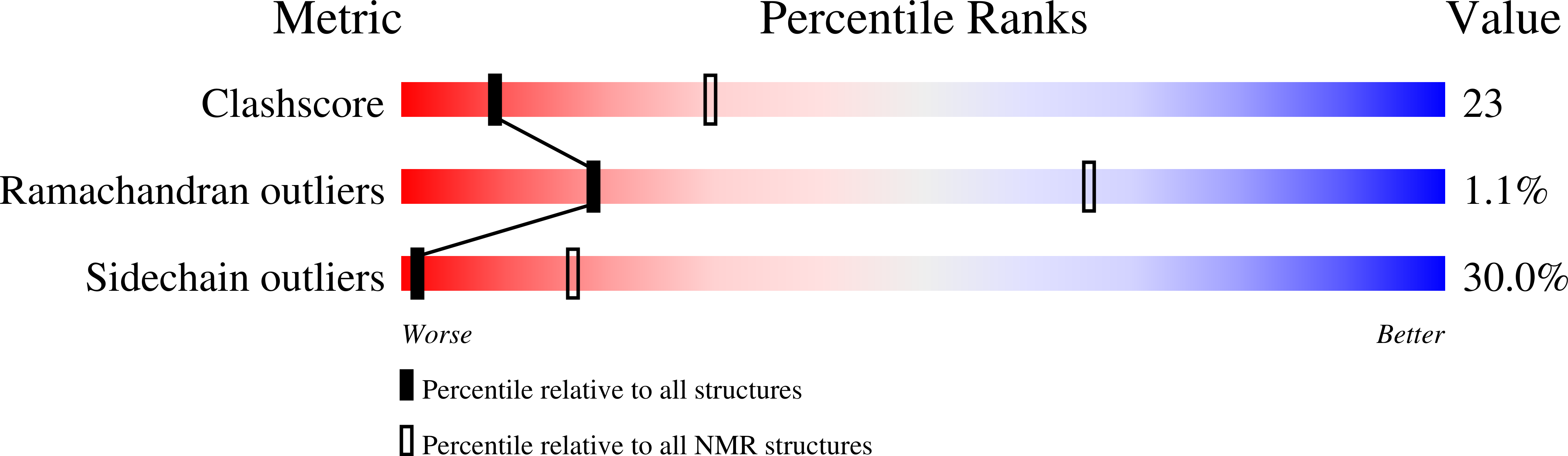Solution structure of the satiety factor, CART, reveals new functionality of a well-known fold.
Ludvigsen, S., Thim, L., Blom, A.M., Wulff, B.S.(2001) Biochemistry 40: 9082-9088
- PubMed: 11478874
- DOI: https://doi.org/10.1021/bi010433u
- Primary Citation of Related Structures:
1HY9 - PubMed Abstract:
Cocaine and amphetamine regulated transcript (CART) peptide has been shown to be an anorectic peptide that inhibits both normal and starvation-induced feeding and completely blocks the feeding response induced by neuropeptide Y and regulated by leptin in the hypothalamus. The C-terminal part containing the three disulfide bridges CART(48-89) is the biologically active part of the molecule affecting food intake. The solution structure of the active part of CART has a fold equivalent to other functionally distinct small proteins. CART consists mainly of turns and loops spanned by a compact framework composed by a few small stretches of antiparallel beta-sheet common to cystine knots.
Organizational Affiliation:
Novo Nordisk A/S, Novo Allé 1, DK-2880 Bagsvaerd, Denmark. svl@novonordisk.com