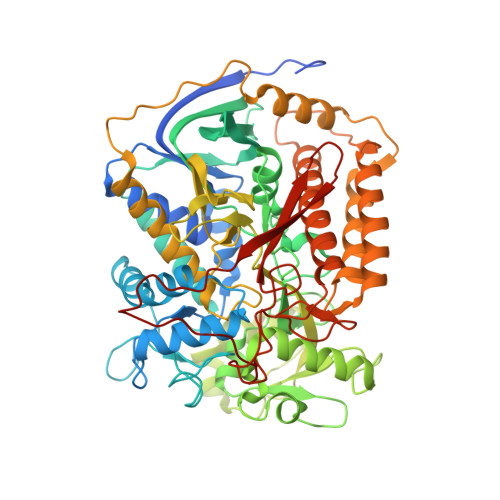
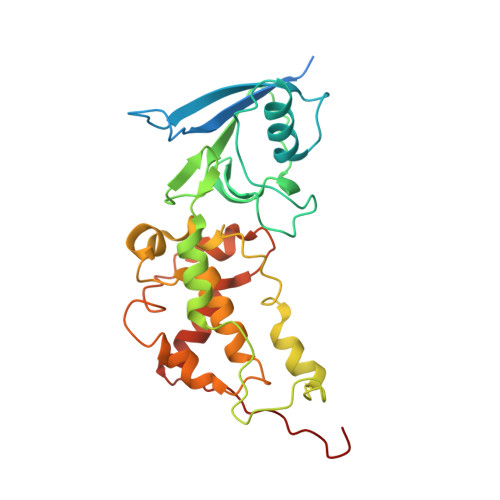
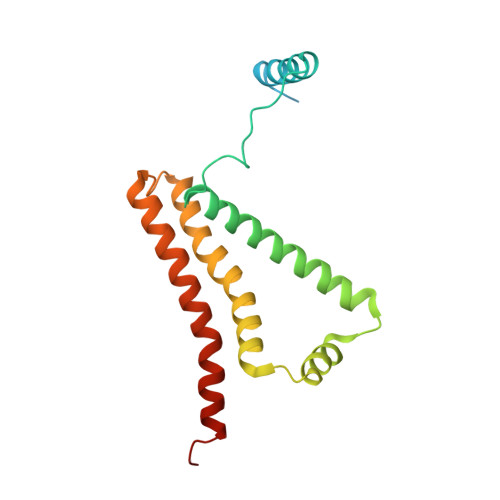
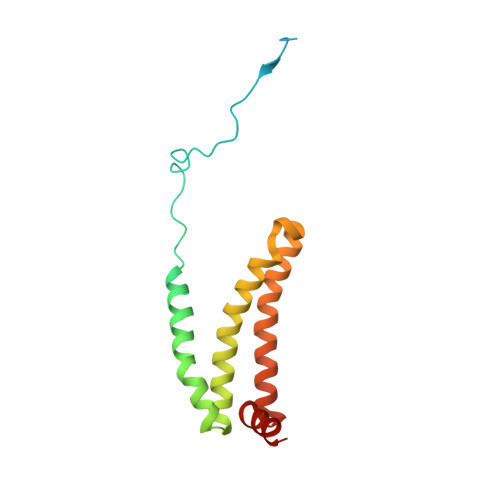
FAD
Query on FAD
Download Ideal Coordinates CCD File
| J [auth A],
R [auth E] | FLAVIN-ADENINE DINUCLEOTIDE
C27 H33 N9 O15 P2
VWWQXMAJTJZDQX-UYBVJOGSSA-N |  | |
EPH
Query on EPH
Download Ideal Coordinates CCD File
| P [auth D],
X [auth H] | L-ALPHA-PHOSPHATIDYL-BETA-OLEOYL-GAMMA-PALMITOYL-PHOSPHATIDYLETHANOLAMINE
C39 H68 N O8 P
MABRTXOVHMDVAT-AAEGOEIASA-N |  | |
HEM
Query on HEM
Download Ideal Coordinates CCD File
| N [auth C],
V [auth G] | PROTOPORPHYRIN IX CONTAINING FE
C34 H32 Fe N4 O4
KABFMIBPWCXCRK-RGGAHWMASA-L |  | |
SF4
Query on SF4
Download Ideal Coordinates CCD File
| L [auth B],
T [auth F] | IRON/SULFUR CLUSTER
Fe4 S4
LJBDFODJNLIPKO-UHFFFAOYSA-N |  | |
F3S
Query on F3S
Download Ideal Coordinates CCD File
| M [auth B],
U [auth F] | FE3-S4 CLUSTER
Fe3 S4
FCXHZBQOKRZXKS-UHFFFAOYSA-N |  | |
UQ1
Query on UQ1
Download Ideal Coordinates CCD File
| O [auth C],
W [auth G] | UBIQUINONE-1
C14 H18 O4
SOECUQMRSRVZQQ-UHFFFAOYSA-N |  | |
FES
Query on FES
Download Ideal Coordinates CCD File
| K [auth B],
S [auth F] | FE2/S2 (INORGANIC) CLUSTER
Fe2 S2
NIXDOXVAJZFRNF-UHFFFAOYSA-N |  | |
MLI
Query on MLI
Download Ideal Coordinates CCD File
| I [auth A],
Q [auth E] | MALONATE ION
C3 H2 O4
OFOBLEOULBTSOW-UHFFFAOYSA-L |  | |