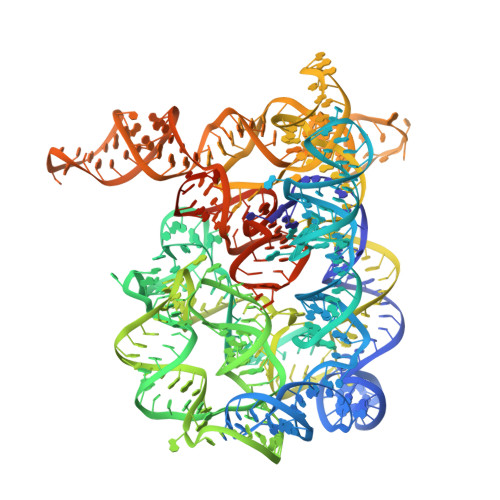
Inclusion of weak high-resolution X-ray data for improvement of a group II intron structure.
Wang, J.(2010) Acta Crystallogr D Biol Crystallogr 66: 988-1000
- PubMed: 20823550
- DOI: https://doi.org/10.1107/S0907444910029938
- Primary Citation of Related Structures:
3G78 - PubMed Abstract:
It is common to report the resolution of a macromolecular structure with the highest resolution shell having an averaged I/sigma(I) > or = 2. Data beyond the resolution thus defined are weak and often poorly measured. The exclusion of these weak data may improve the apparent statistics and also leads to claims of lower resolutions that give some leniency in the acceptable quality of refined models. However, the inclusion of these data can provide additional strong constraints on atomic models during structure refinement and thus help to correct errors in the original models, as has recently been demonstrated for a protein structure. Here, an improved group II intron structure is reported arising from the inclusion of these data, which helped to define more accurate solvent models for density modification during experimental phasing steps. With the improved resolution and accuracy of the experimental phases, extensive revisions were made to the original models such that the correct tertiary interactions of the group II intron that are essential for understanding the chemistry of this ribozyme could be described.
Organizational Affiliation:
Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06520, USA. jimin.wang@yale.edu