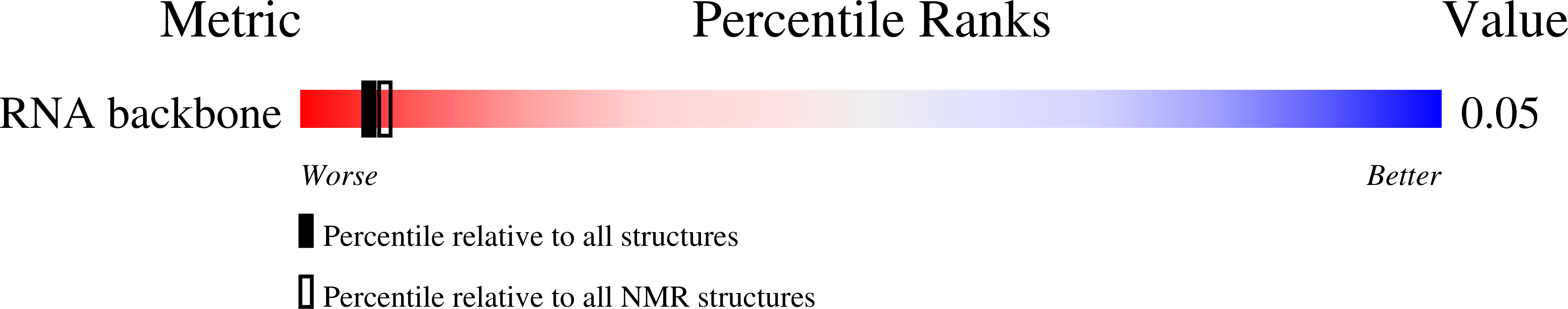Frameshifting RNA pseudoknots: Structure and mechanism.
Giedroc, D.P., Cornish, P.V.(2009) Virus Res 139: 193-208
- PubMed: 18621088
- DOI: https://doi.org/10.1016/j.virusres.2008.06.008
- Primary Citation of Related Structures:
2RP0, 2RP1 - PubMed Abstract:
Programmed ribosomal frameshifting (PRF) is one of the multiple translational recoding processes that fundamentally alters triplet decoding of the messenger RNA by the elongating ribosome. The ability of the ribosome to change translational reading frames in the -1 direction (-1 PRF) is employed by many positive strand RNA viruses, including economically important plant viruses and many human pathogens, such as retroviruses, e.g., HIV-1, and coronaviruses, e.g., the causative agent of severe acute respiratory syndrome (SARS), in order to properly express their genomes. -1 PRF is programmed by a bipartite signal embedded in the mRNA and includes a heptanucleotide "slip site" over which the paused ribosome "backs up" by one nucleotide, and a downstream stimulatory element, either an RNA pseudoknot or a very stable RNA stem-loop. These two elements are separated by six to eight nucleotides, a distance that places the 5' edge of the downstream stimulatory element in direct contact with the mRNA entry channel of the 30S ribosomal subunit. The precise mechanism by which the downstream RNA stimulates -1 PRF by the translocating ribosome remains unclear. This review summarizes the recent structural and biophysical studies of RNA pseudoknots and places this work in the context of our evolving mechanistic understanding of translation elongation. Support for the hypothesis that the downstream stimulatory element provides a kinetic barrier to the ribosome-mediated unfolding is discussed.
Organizational Affiliation:
Department of Chemistry, Indiana University, 212 S. Hawthorne Drive, Bloomington, IN 47405-7102, USA. giedroc@indiana.edu