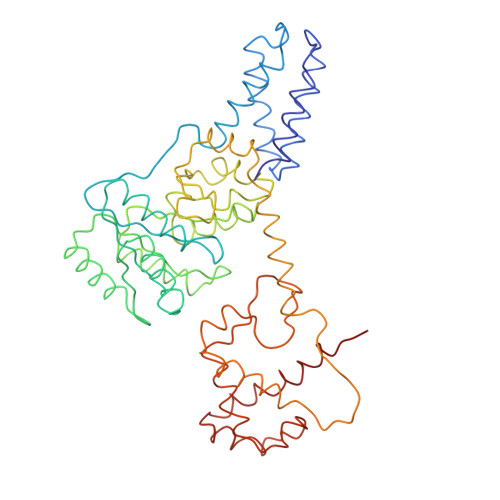
Structure of the E. Coli Signal Recognition Particle Bound to a Translating Ribosome
Schaffitzel, C., Oswald, M., Berger, I., Ishikawa, T., Abrahams, J.P., Koerten, H.K., Koning, R.I., Ban, N.(2006) Nature 444: 503
- PubMed: 17086205
- DOI: https://doi.org/10.1038/nature05182
- Primary Citation of Related Structures:
2IY3 - PubMed Abstract:
The prokaryotic signal recognition particle (SRP) targets membrane proteins into the inner membrane. It binds translating ribosomes and screens the emerging nascent chain for a hydrophobic signal sequence, such as the transmembrane helix of inner membrane proteins. If such a sequence emerges, the SRP binds tightly, allowing the SRP receptor to lock on. This assembly delivers the ribosome-nascent chain complex to the protein translocation machinery in the membrane. Using cryo-electron microscopy and single-particle reconstruction, we obtained a 16 A structure of the Escherichia coli SRP in complex with a translating E. coli ribosome containing a nascent chain with a transmembrane helix anchor. We also obtained structural information on the SRP bound to an empty E. coli ribosome. The latter might share characteristics with a scanning SRP complex, whereas the former represents the next step: the targeting complex ready for receptor binding. High-resolution structures of the bacterial ribosome and of the bacterial SRP components are available, and their fitting explains our electron microscopic density. The structures reveal the regions that are involved in complex formation, provide insight into the conformation of the SRP on the ribosome and indicate the conformational changes that accompany high-affinity SRP binding to ribosome nascent chain complexes upon recognition of the signal sequence.
Organizational Affiliation:
ETH Zurich, Institute for Molecular Biology and Biophysics, HPK Building, Schafmattstrasse 20, 8093 Zurich, Switzerland.