Solution structure of the GUCT domain from human RNA helicase II/Gubeta reveals the RRM fold, but implausible RNA interactions
Ohnishi, S., Paakkonen, K., Koshiba, S., Tochio, N., Sato, M., Kobayashi, N., Harada, T., Watanabe, S., Muto, Y., Guntert, P., Tanaka, A., Kigawa, T., Yokoyama, S.(2008) Proteins 74: 133-144
- PubMed: 18615715
- DOI: https://doi.org/10.1002/prot.22138
- Primary Citation of Related Structures:
2E29 - PubMed Abstract:
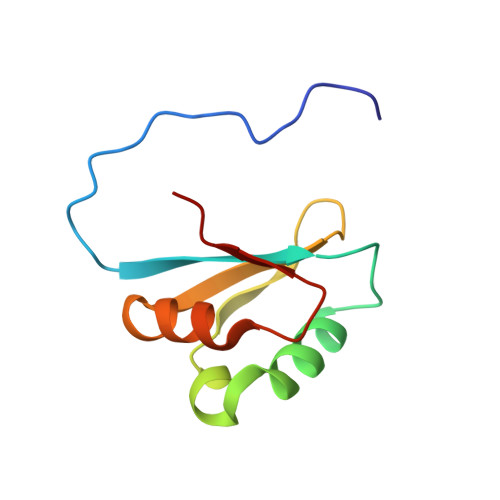
Human RNA helicase II/Gu alpha (RH-II/Gu alpha) and RNA helicase II/Gu beta (RH-II/Gu beta) are paralogues that share the same domain structure, consisting of the DEAD box helicase domain (DEAD), the helicase conserved C-terminal domain (helicase_C), and the GUCT domain. The N-terminal regions of the RH-II/Gu proteins, including the DEAD domain and the helicase_C domain, unwind double-stranded RNAs. The C-terminal tail of RH-II/Gu alpha, which follows the GUCT domain, folds a single RNA strand, while that of RH-II/Gu beta does not, and the GUCT domain is not essential for either the RNA helicase or foldase activity. Thus, little is known about the GUCT domain. In this study, we have determined the solution structure of the RH-II/Gu beta GUCT domain. Structural calculations using NOE-based distance restraints and residual dipolar coupling-based angular restraints yielded a well-defined structure with beta-alpha-alpha-beta-beta-alpha-beta topology in the region for K585-A659, while the Pfam HMM algorithm defined the GUCT domain as G571-E666. This structure-based domain boundary revealed false positives in the sequence homologue search using the HMM definition. A structural homology search revealed that the GUCT domain has the RRM fold, which is typically found in RNA-interacting proteins. However, it lacks the surface-exposed aromatic residues and basic residues on the beta-sheet that are important for the RNA recognition in the canonical RRM domains. In addition, the overall surface of the GUCT domain is fairly acidic, and thus the GUCT domain is unlikely to interact with RNA molecules. Instead, it may interact with proteins via its hydrophobic surface around the surface-exposed tryptophan.
Organizational Affiliation:
Systems and Structural Biology Center, RIKEN, Tsurumi, Yokohama 230-0045, Japan.