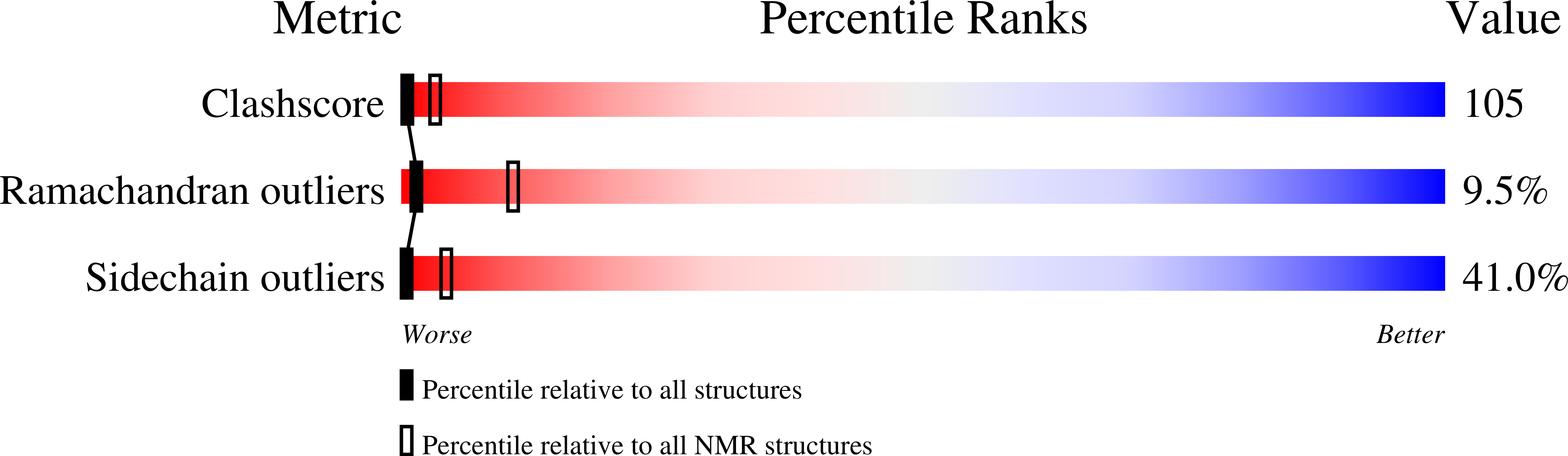High-resolution structure of the histidine-containing phosphocarrier protein (HPr) from Staphylococcus aureus and characterization of its interaction with the bifunctional HPr kinase/phosphorylase
Maurer, T., Meier, S., Kachel, N., Munte, C.E., Hasenbein, S., Koch, B., Hengstenberg, W., Kalbitzer, H.R.(2004) J Bacteriol 186: 5906-5918
- PubMed: 15317796
- DOI: https://doi.org/10.1128/JB.186.17.5906-5918.2004
- Primary Citation of Related Structures:
1KA5 - PubMed Abstract:
A high-resolution structure of the histidine-containing phosphocarrier protein (HPr) from Staphylococcus aureus was obtained by heteronuclear multidimensional nuclear magnetic resonance (NMR) spectroscopy on the basis of 1,766 structural restraints. Twenty-three hydrogen bonds in HPr could be directly detected by polarization transfer from the amide nitrogen to the carbonyl carbon involved in the hydrogen bond. Differential line broadening was used to characterize the interaction of HPr with the HPr kinase/phosphorylase (HPrK/P) of Staphylococcus xylosus, which is responsible for phosphorylation-dephosphorylation of the hydroxyl group of the regulatory serine residue at position 46. The dissociation constant Kd was determined to be 0.10 +/- 0.02 mM at 303 K from the NMR data, assuming independent binding. The data are consistent with a stoichiometry of 1 HPr molecule per HPrK/P monomer in solution. Using transversal relaxation optimized spectroscopy-heteronuclear single quantum correlation, we mapped the interaction site of the two proteins in the 330-kDa complex. As expected, it covers the region around Ser46 and the small helix b following this residue. In addition, HPrK/P also binds to the second phosphorylation site of HPr at position 15. This interaction may be essential for the recognition of the phosphorylation state of His15 and the phosphorylation-dependent regulation of the kinase/phosphorylase activity. In accordance with this observation, the recently published X-ray structure of the HPr/HPrK core protein complex from Lactobacillus casei shows interactions with the two phosphorylation sites. However, the NMR data also suggest differences for the full-length protein from S. xylosus: there are no indications for an interaction with the residues preceding the regulatory Ser46 residue (Thr41 to Lys45) in the protein of S. xylosus. In contrast, it seems to interact with the C-terminal helix of HPr in solution, an interaction which is not observed for the complex of HPr with the core of HPrK/P of L. casei in crystals.
Organizational Affiliation:
Institut für Biophysik und Physikalische Biochemie, Universität Regensburg, Regensburg, Germany.